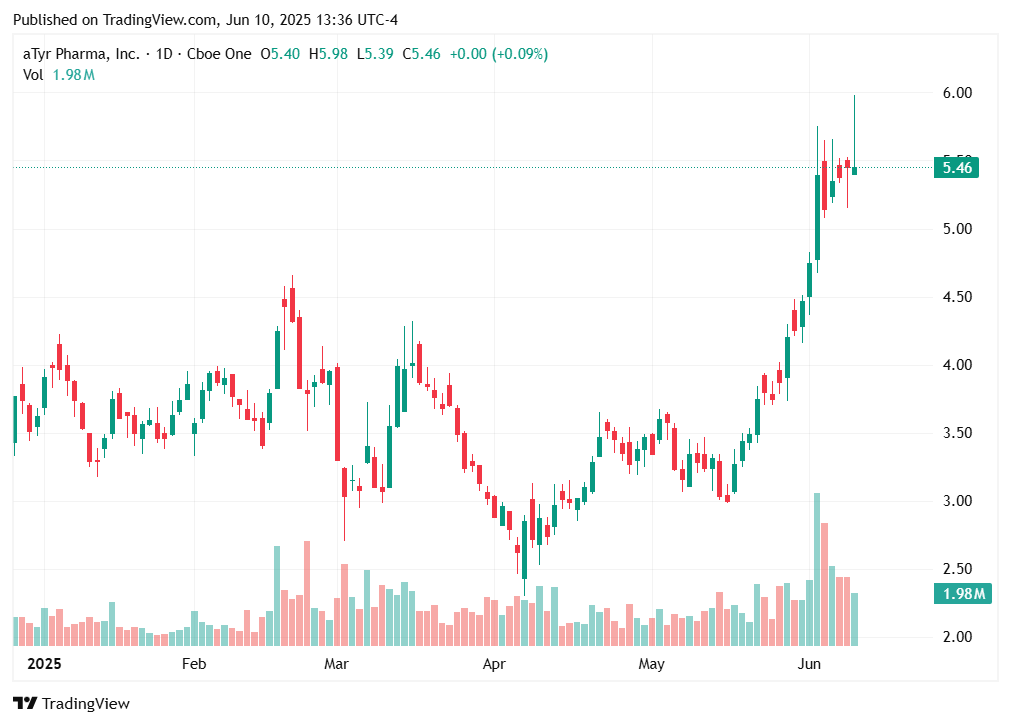
aTyr Pharma's (ATYR) efzofitimod aims to get in front of pulmonary sarcoidosis, lung disease
Assigning a 62.5% overall probability aTyr Pharma will report positive topline data from a phase III trial testing its drug efzofitimod to treat pulmonary sarcoidosis.
Patients experience pulmonary sarcoidosis after developing granulomas, which are small clumps of inflammatory cells. Granulomas form as a reaction to infections, inflammation, irritants or foreign objects; the clump attempts to contain the perceived threat from, for example, an infection or an irritant. While the clumps can appear in the skin or other tissues, they often appear in the lungs, triggering a condition known as pulmonary sacrodiosis.
Over a period of time, the inflammation and scarring in the lungs initiated by the granulomas can lead to difficult breathing and poor lung function. Pulmonary sacrodiosis is the most prevalent form of interstitial lung disease and primarily is treated with oral corticosteroids. aTyr Pharma, Inc. (NASDAQ:ATYR) is applying its tRNA (transfer RNA) synthetase biology platform to pulmonary sarcoidosis, seeking to intervene prior to a series of lung diseases that can follow the scarring caused by pulmonary sarcoidosis. No new treatments have been approved for pulmonary sarcoidosis in more than 70 years. We assigns a 62.5% overall probability aTyr Pharma will report positive topline data from a phase III trial testing its drug efzofitimod to treat pulmonary sarcoidosis.
Key catalysts
Topline phase III data from EFZO-FIT trial testing efzofitimod in pulmonary sarcoidosis
Key catalyst dates
3Q 2025
Overall Probability: 62.5%
Market Demand: 62.5%
Adoption by KOLs: 66.7%
Clinical Trial Progress: 66.7%
Experience & Capital: 50.0%
aTyr Pharma’s synthetase biology platform is based on a discovery by the company’s founder, Paul Schimmel, that a protein from one tRNA synthetase gene could work as an extracellular modulator of angiogenesis (cell death). Further refinement of the science led to the conclusion tRNA synthetases could influence cellular stress and tissue homeostasis in certain diseases. In pulmonary sacrodiosis, efzofitimod attempts to tamp down the cellular stress caused by the scarring in the air sacs and small airways of the lungs.
aTyr Pharma has created a portfolio covering more than 300 protein compositions derived from all 20 tRNA synthetase genes to treat selected pathways affected by extracellular tRNA synthetases. Efzofitimod is the company’s most advanced drug.
In a phase Ib/IIa trial, the company tested efzofitimod for safety and dosing but also to assess whether 37 pulmonary sarcoidosis patients could be weaned off or reduce their use of corticosteroids to manage their conditions. Three efzofitimod doses – 1 mg/kg, 3 mg/kg and 5 mg/kg – were administered monthly in the double-blind, placebo-controlled study over a six-month period.
Efzofitimod demonstrated it was safe and well tolerated and a steroid-sparing effect was observed most dramatically when 33% of the patients in the 5.0 mg/kg group were able to completely taper off corticosteroids. The trial also demonstrated improvement in forced vital capacity (FVC) in the lungs as well as improvements in time to first relapse and relapse rates for corticosteroid use in patients receiving the higher doses (3.0 and 5.0 mg/kg) compared to the lower dose (1.0 mg/kg).
THE EDGE
Following the phase Ib/IIa trial, aTyr Pharma designed the phase III trial, named EFZO-FIT, to replicate the steroid-sparing endpoint. The primary outcome measure is the change from baseline in mean daily oral corticosteroid dose. The phase III trial will last 48 weeks in contrast to the 26-week term of the previous trial. Only two doses, 3.0 mg/kg and 5.0 mg/kg, will be tested as well as a placebo arm. Investigators enrolled 268 patients at 85 centers in 9 countries.
The steroid-sparing endpoint appears to be highly achievable in pulmonary sacrodiosis. Normal clinical trial practice calls for testing several doses of the drug during phase II, as aTyr Pharma did during the phase Ib/IIa. The results revealed efzofitimod was safe at the higher two doses. Thus, the phase III trial will attempt replicate the effect of the higher doses in a larger patient pool. The probability of achieving the steroid-sparing effect generally increases with more patients evaluated. aTyr Pharma enrolled 268 patients ahead of schedule in contrast to the 37 total in the prior trial. Moreover, no patients will receive the 1.0 mg/kg dose in the phase III trial.
The phase III trial design incorporates a forced steroid taper. Accordingly, aTyr Pharma only needs to demonstrate efzofitimod reduces patient dependence on corticosteroids. There are no requirements to demonstrate, for example, improved breathing or lung function.
Overuse of corticosteroids can lead to high blood pressure, increased blood sugar and osteoporosis, as well as difficult breathing and poor lung function. aTyr Pharma’s straight-line phase III design points to success in treating pulmonary sarcoidosis, for which no specific drug is indicated.
Disclosures:
Initiating coverage and a position in ATYR, and may close position prior to or following the expected catalyst date.




Nicely written