SanaCurrents on Verrica's drug-device combo to become the first treatment for benign skin disease in children | $VRCA
SUMMARY
Deep sea fishermen chase marlins and bluefin tuna. Big dermatology companies chase market share in aesthetics (think Botox), psoriasis and acne, even though effective treatments are rare in the latter two indications. The dermatology company Verrica Pharmaceuticals Inc. (NASDAQ:VRCA) decided to fish in a lake with its drug-device combination treatment VP-102.
VP-102 contains a controlled formulation of the approved burn agent cantharidin (0.7% w/v). While Verrica expects VP-102 can be more effective than current cantharidin treatments to heal external genital warts and common warts the first market indication for VP-102 likely will be molluscum contagiosum, a benign skin disease. The FDA recently set July 23, 2023, as the PDUFA date for VP-102 to treat molluscum contagiosum (MC).
Next Key catalysts
PDUFA decision for VP-102 to treat the skin disease molluscum contagiosum
Key catalyst dates
July 23, 2023
Sentiment, MACE Score & The Edge….
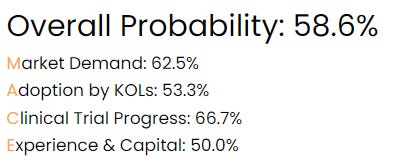
SanaCurrents assigns a 58.6% overall probability the FDA will sign off on VP-102.
*Note: This table appeared on https://sanacurrents.com first. It is being offered as a resource to as part of our effort to supplement this Substack with posts prior to the launch of Biotech Currents. Therefore, some data may appear more current than the post date due to already completed catalysts.
MC strikes approximately six million people in the US annually, according to Verrica. Similar to warts, small, painless lesions appear on the body as round, firm bumps. Unlike warts, however, the molluscum infection results from transmission of a poxvirus (MC virus) and primarily strikes children.
The bumps can disappear once the virus passes through the body but as children experience the condition, they are likely to scratch the bumps and transmit the virus to siblings or other children by touching toys and common surfaces. Some people infected experience MC for months or even up to a year. Without treatment, MC can last for an average of 13 months, and in some cases, up to several years, according to Verrica. There currently are no FDA-approved treatments for MC.
The drug-device combination of VP-102 consists of a single-use applicator to enable precise topical dosing to the scattered lesions. Verrica tested VP-102 in a total of 528 patients between the ages of 2 and 16 in two, similar phase III trials named CAMP-1 and CAMP-2. The primary efficacy endpoint was the proportion of VP-102–treated participants achieving complete clearance of all lesions (baseline and new) compared to participants treated with the control vehicle at the end-of-study visit on day 84.
In CAMP-1, treatment with VP-102 demonstrated superior efficacy to the vehicle control in with 46.3% complete clearance of MC lesions at the end of the study visit versus 17.9% for the vehicle control (p < 0.001), In CAMP-2, the figures were 54.0% for the treatment arm versus 13.4% for the vehicle control (VP-102: 54.0% vs 13.4% (p < 0.001). Surprisingly, the phase III trials were completed in 2019.
THE EDGE
Verrica received two complete response letters (CRLs) for VP-102. The first was delivered in July 2020 and the second in September 2021. It is important to recognize both of the CRLs were sent back to Verrica at a time when the FDA staff was overwhelmed with more pressing Covid19 commitments.
In the July 2020 CRL, the FDA requested more information regarding the Chemistry, Manufacturing, and Controls (CMC) process for VP-102, as well as Human Factors validation. The FDA did not identify any clinical deficiencies in clinical studies or efficacy outcomes for VP-102. After Verrica answered the CMC questions, the FDA in September 2021 wrote to Verrica and said it identified deficiencies at a facility of a contract manufacturing organization (CMO) used by the company. The deficiencies were not specifically related to the manufacturing of VP-102. Rather, the concerns regarded general quality issues at the facility, according to Verrica.
Two CRLS would appear to signal Verrica poorly managed the NDA process at the FDA. CMC deficiencies, however, appear to be cited frequently by the agency to delay decisions in which it has not had enough time to review. The practice also appears to be applied more frequently to small cap companies as NDAs from smaller companies tend to go to the bottom of the in box. Small companies like Verrica also rely more on outside CMOs and those CMOs likewise may incur the wrath of FDA more frequently.
With the acceptance of the VP-102 NDA on February 27, 2023, Verrica appears to have answered all the FDA concerns and emerged from the regulatory storm it experienced over the past 30 months. The third NDA filing is not guaranteed to be a success but it also does not signal a strikeout as Verrica responded to all the non-clinical issues raised by the FDA.
The delay in VP-102 reaching the market falls more at the feet of an understaffed FDA than it does the drug’s efficacy or the company’s efforts to work with the FDA.
Disclosure:
SanaCurrents, the parent of BioCurrents, is initiating coverage and a position in VRCA. SanaCurrents may close its position in VRCA prior to or following the expected catalyst date.